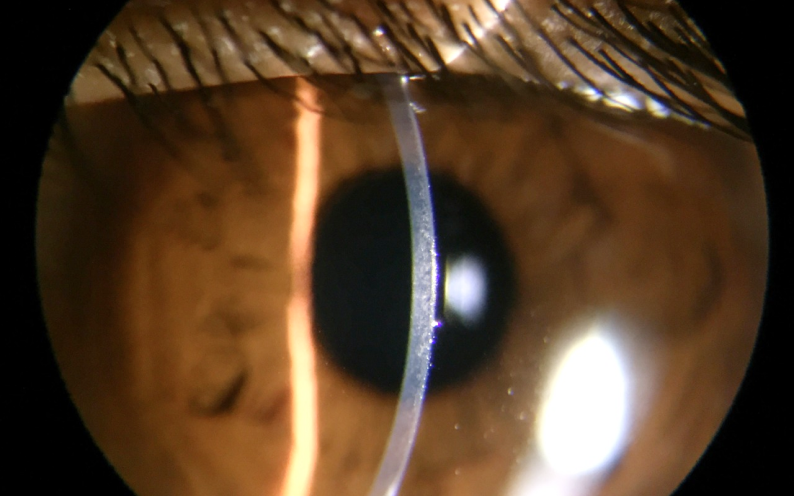
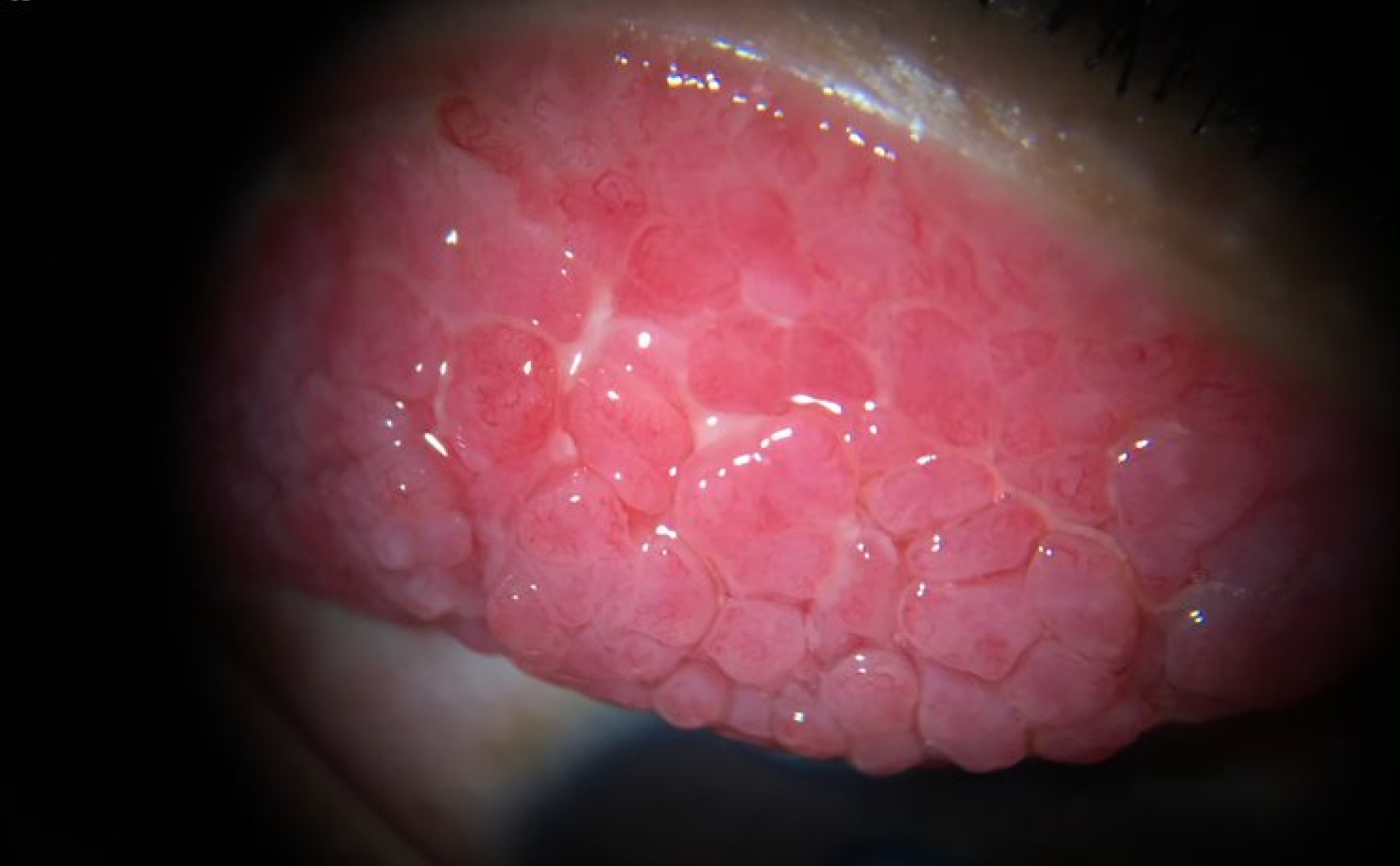
See. Capture. Photo-document.
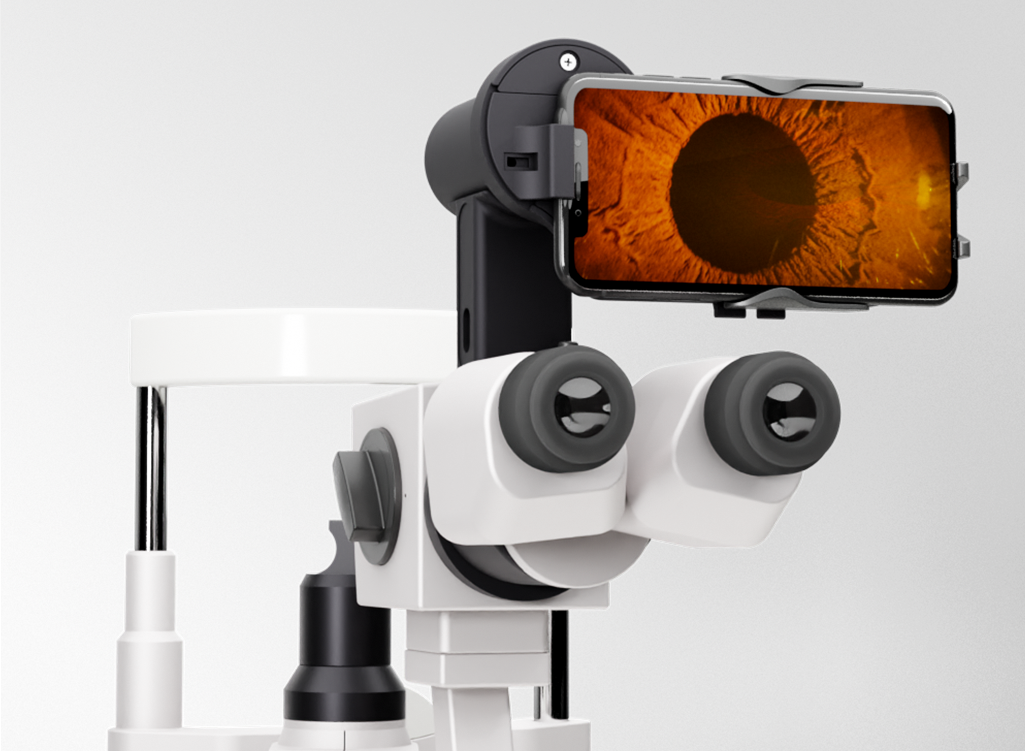
Remidio Anterior Imaging Module (AIM)
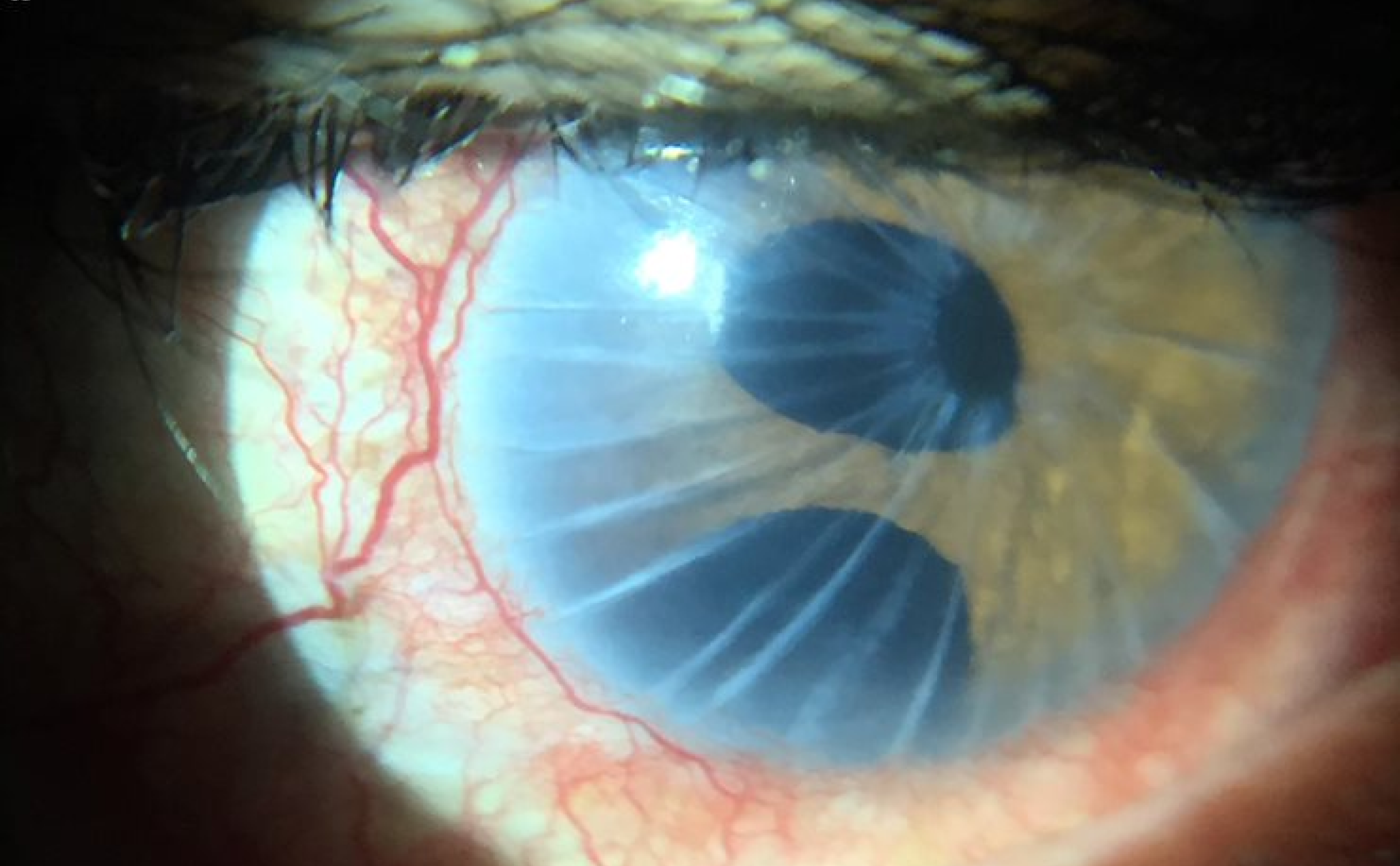
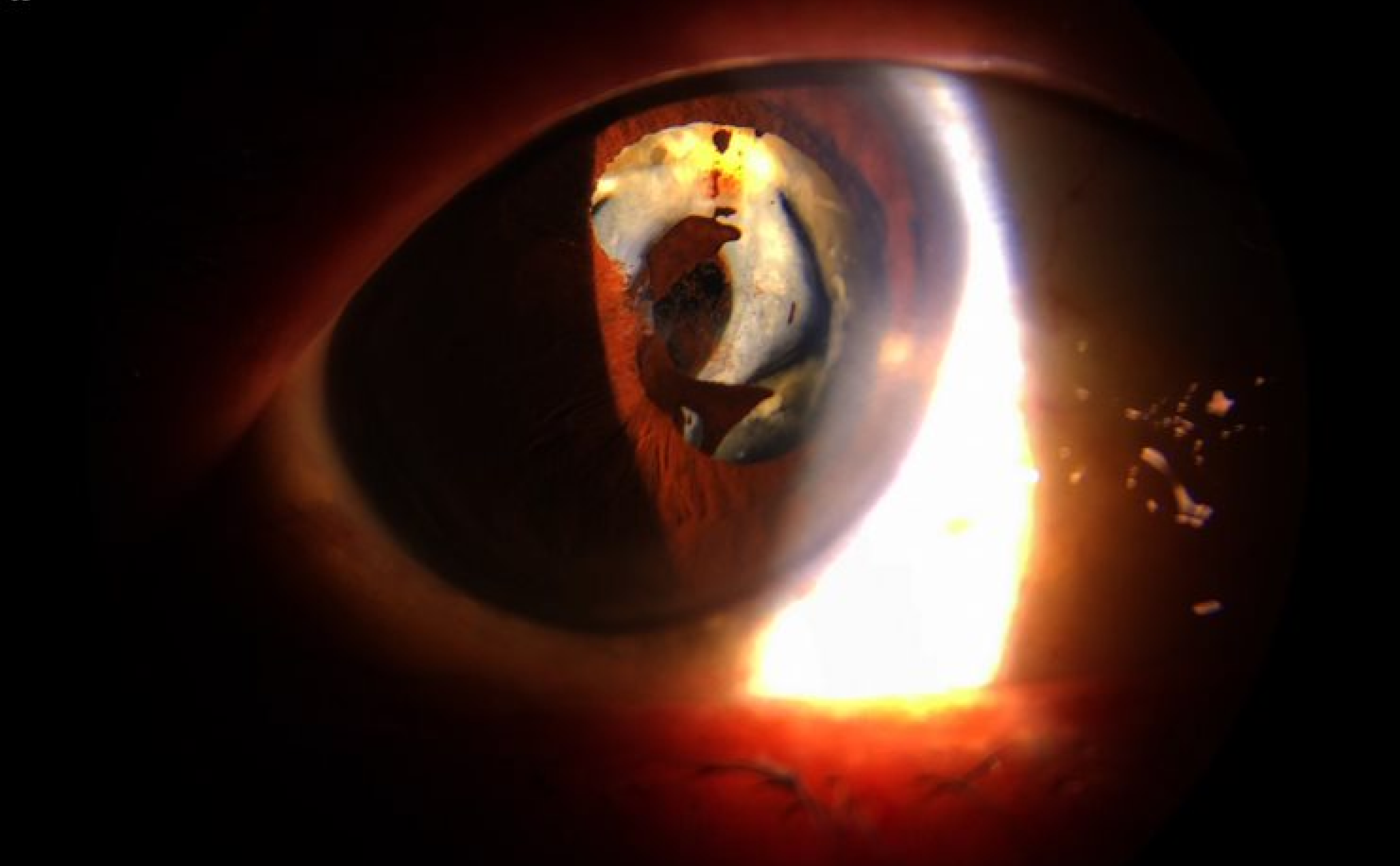
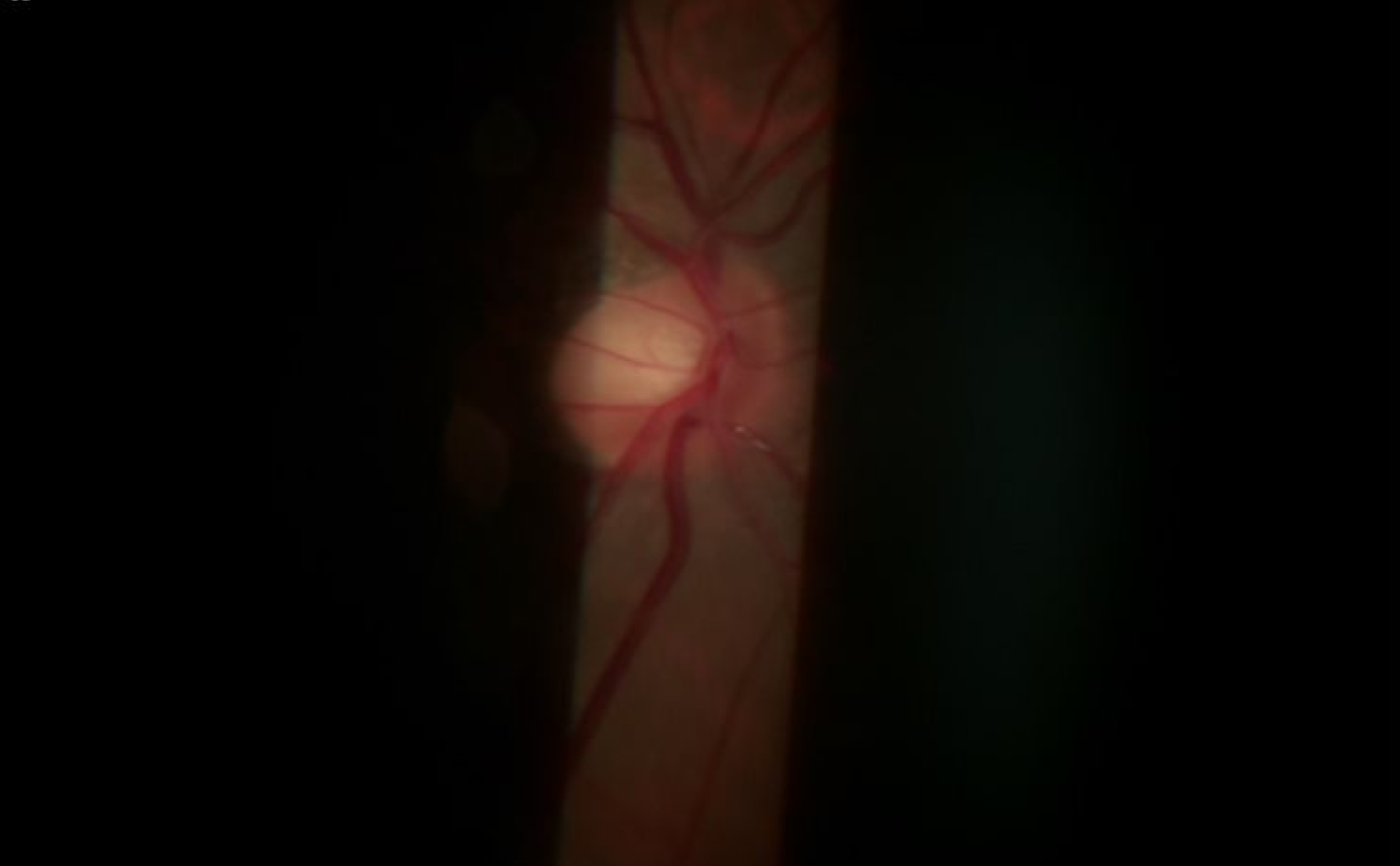
Capture Digital Images even as you simultaneously observe on the eyepiece. What you see, is what you document.
Capture Digital Images even as you simultaneously observe on the eyepiece. What you see, is what you document.