Comprehensive. Automated. Intelligent.
VX 650
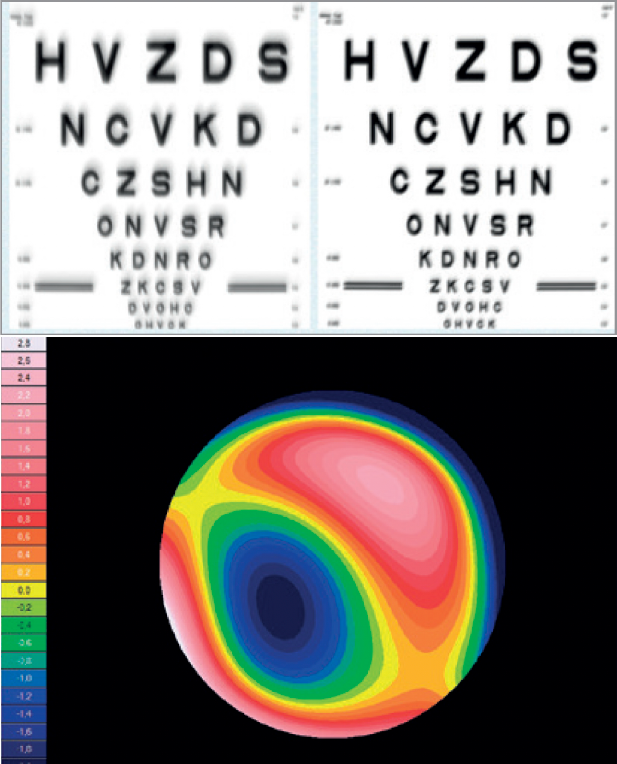
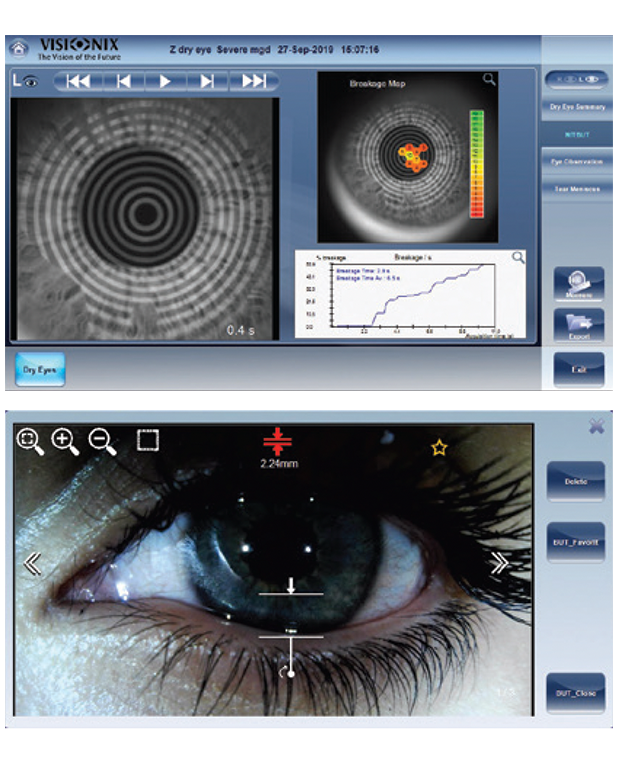
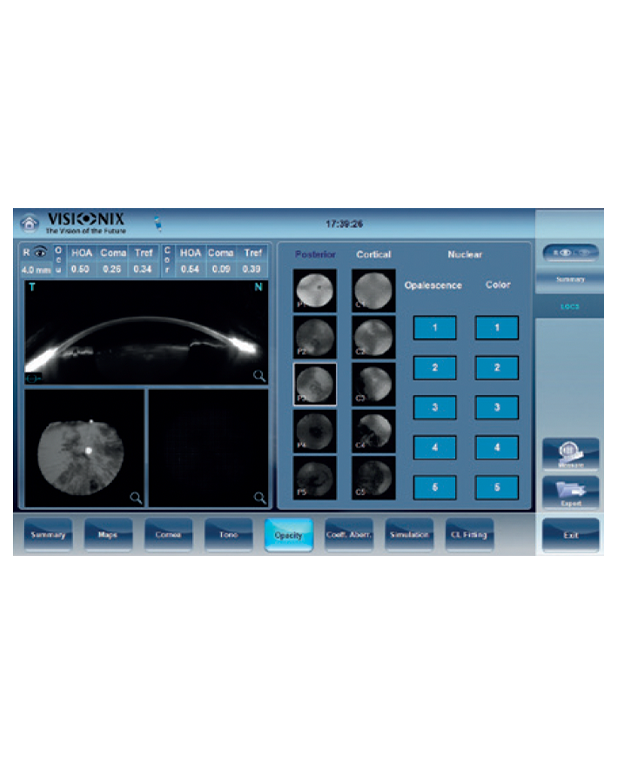
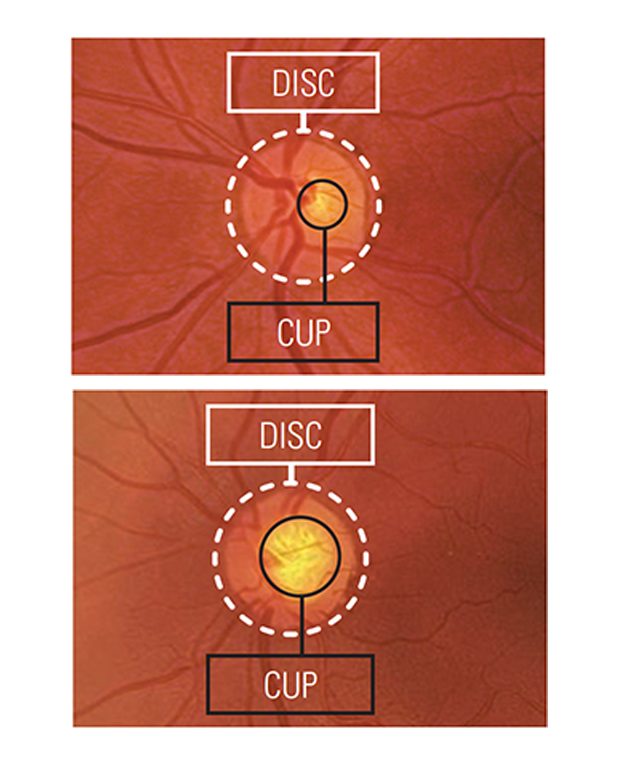
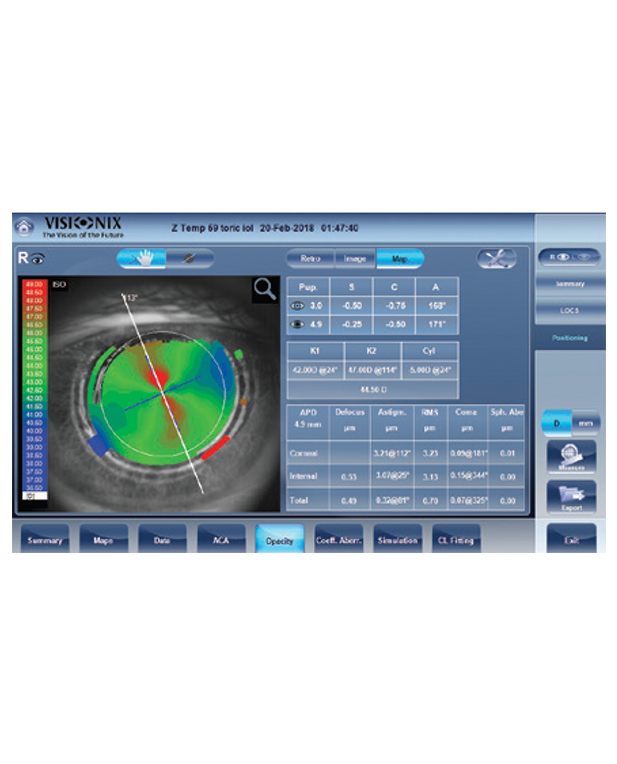
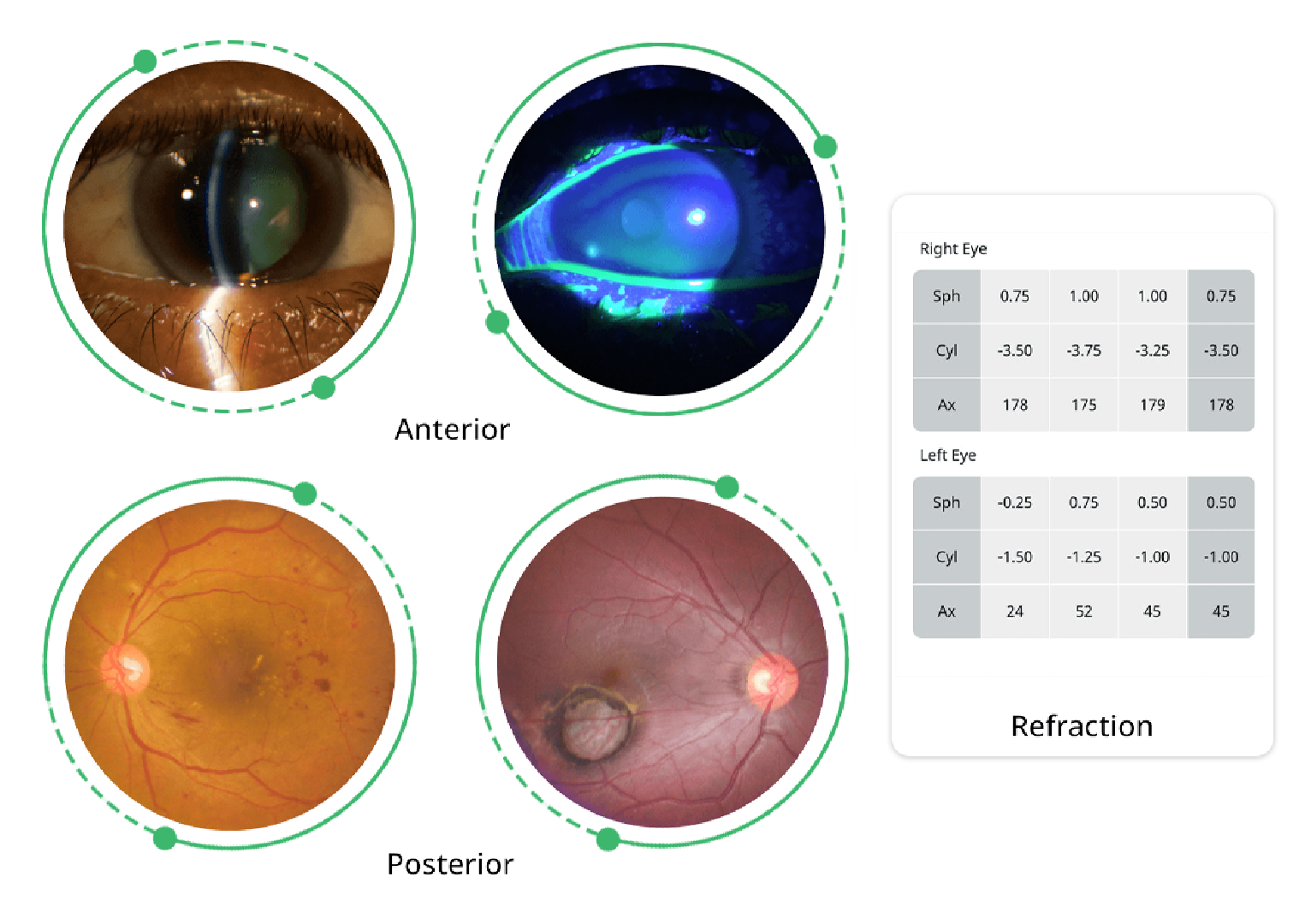
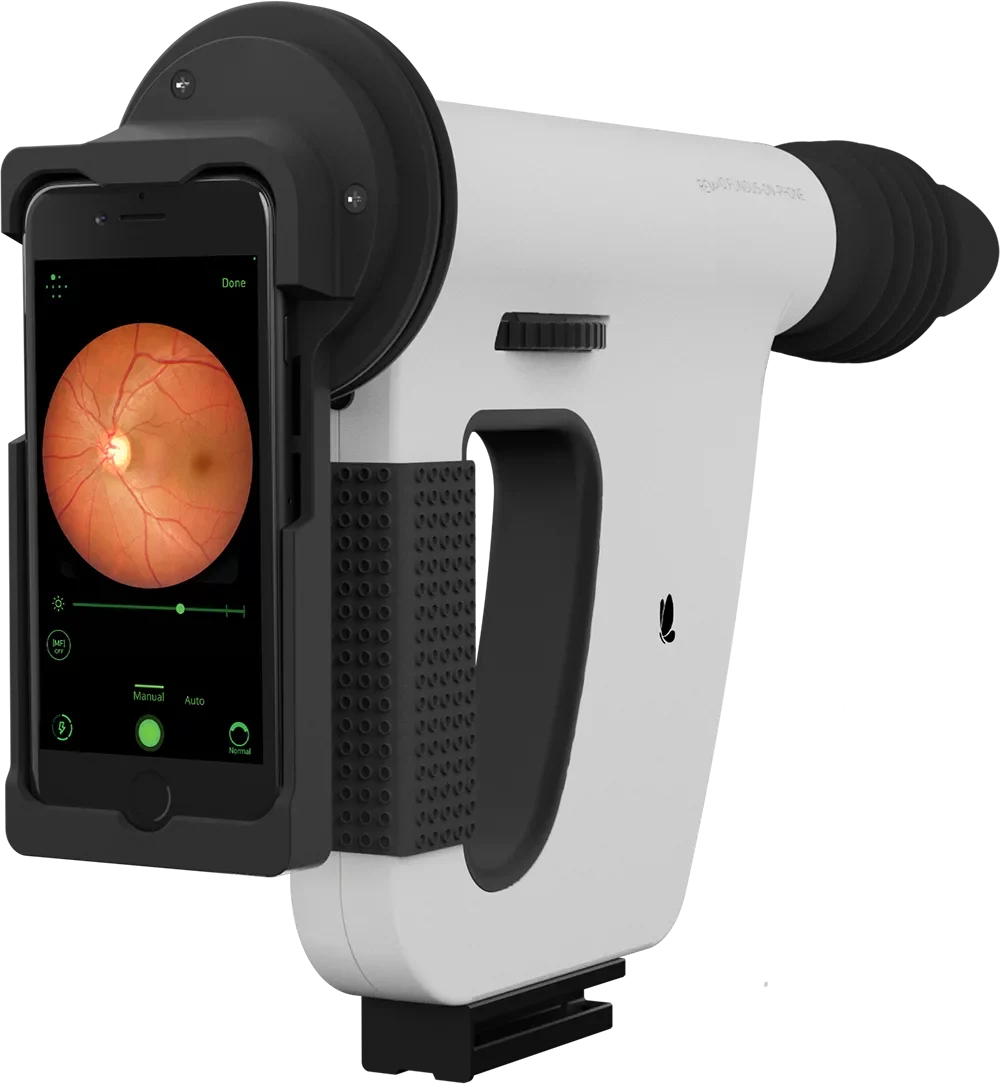
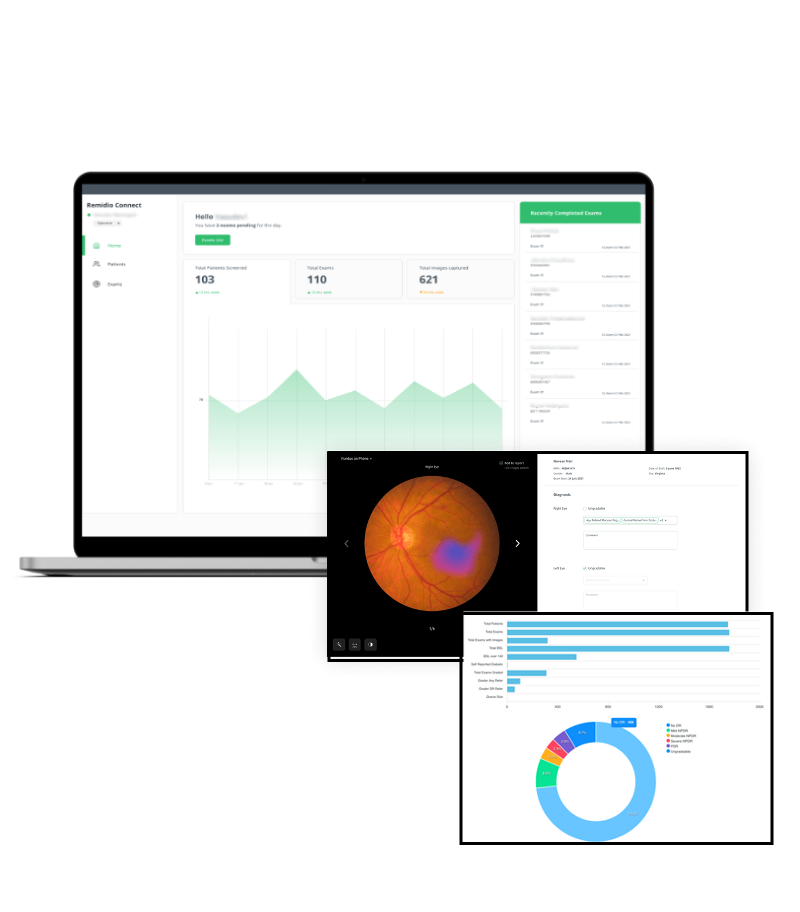
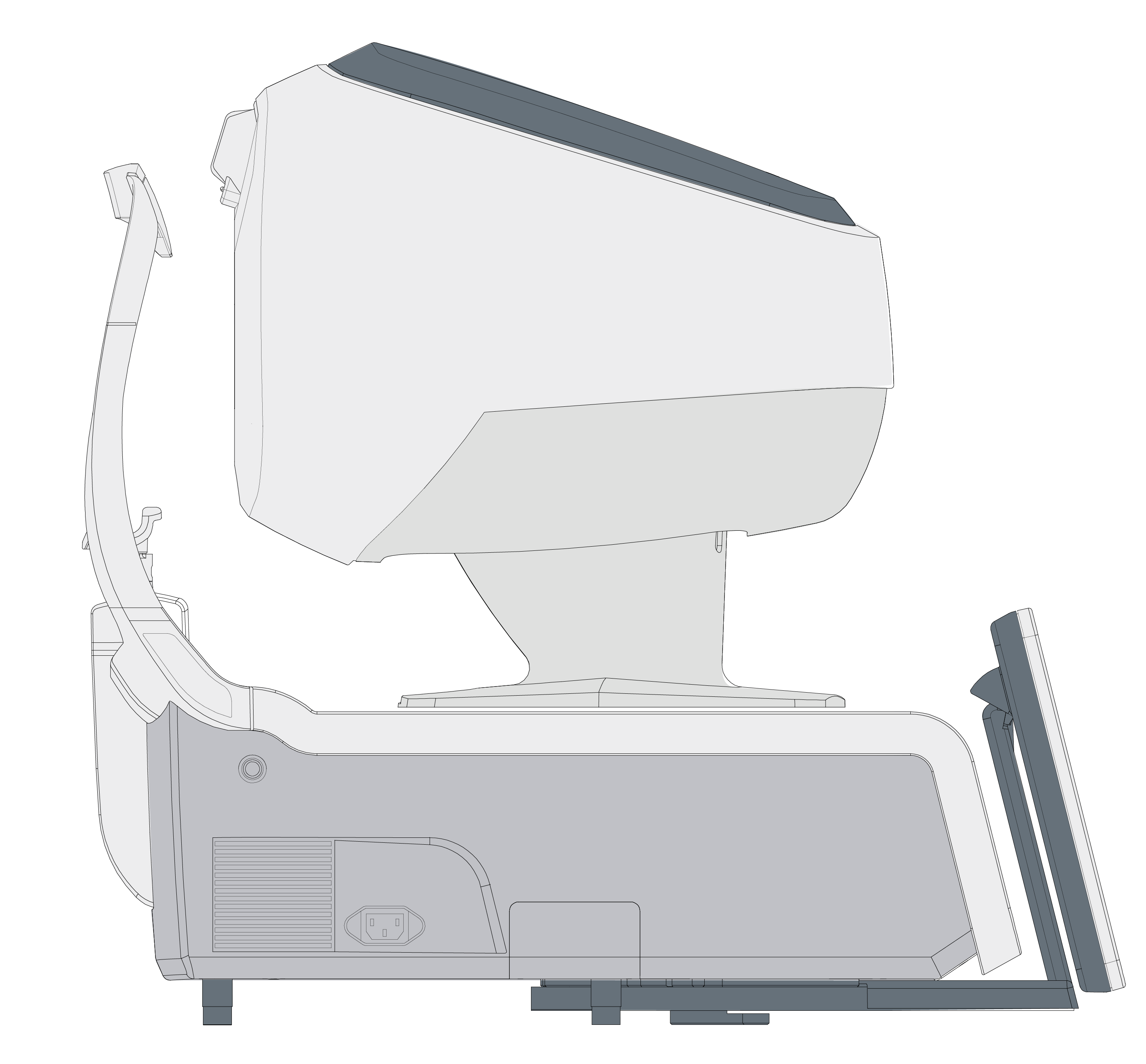
The only fully automated device that performs a complete anterior and posterior eye exam, co-created by Remidio and Visionix. It combines refraction, aberrometry, corneal topography, iridocorneal angle assessment, pachymetry, dry eye evaluation, tonometry, and non-mydriatic fundus imaging in a single workflow, delivering fast, standardized, and repeatable results for both eyes in under three minutes.